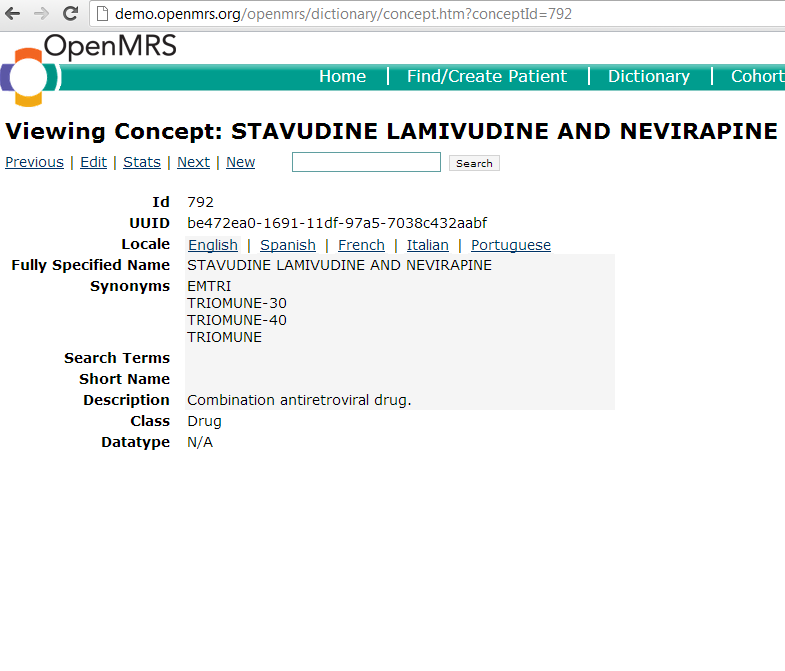
A drug concept is any concept with a "drug" class. The "drug" class should only be assigned to concepts that are drugs/medications. It is appropriate to create a drug concept anytime an implementation needs to store data about a particular medication. One should be sure to include all possible synonyms when creating the concept, as drugs oftentimes have many different names by which they may be referred. Once created, that this concept will reside in the concept table and can be used later to create different formularies if needed.
Here is an example of a drug concept:
Drug Formularies
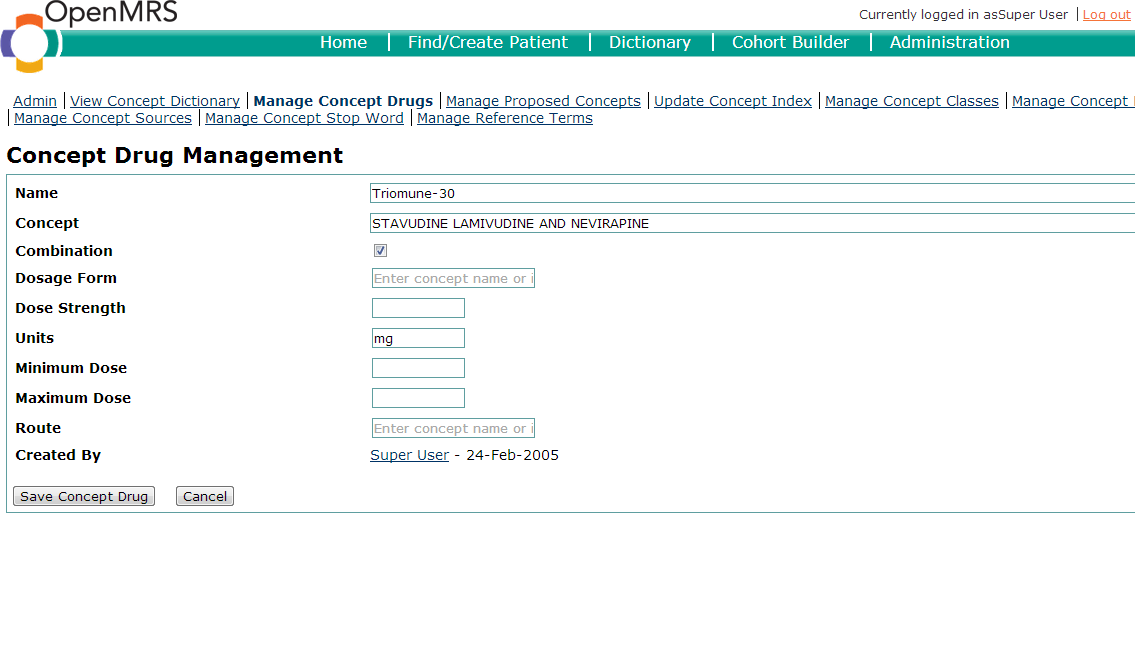
Drugs oftentimes have many different formulations. In OpenMRS, drugs and formulations have a 1:n relationship. In other words, each drug concept may have an unlimited number of drug formulations. Drug formulations may be created using the "Manage Concept Drug" page of the user-interface. When creating a drug formulation, you specify the drug concept to which the formulation is linked.
The following items should be defined in the creation of a drug formulary:
- Name - This is what the concept drug name should be. It is wise to use standard conventions for your implementation.
- Concept - This is the Concept ID which the concept drug is mapped to.
- Combination - Tick this box if the drug is a combination drug.
- Dosage Form - The preparation of the medication. Common forms include: Powder, syrup, tablet, capsule, solution,oral suspension, etc.
- Strength - The strength of the formulary. For example, an ISONIAZID 100 mg TAB would have a strength of 100 mg.
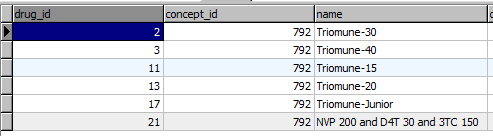
Each each drug formulation shares a concept_id with it's drug concept, but has a unique drug_id. The formulations are all stored in the drug table.
Best practices
Concept names for drugs should represent generic drug names (ideally mapped to RXNORM and SNOMED).
What are naming conventions for drug formularies used by different sites?
Concept | PIH | AMPATH |
|---|---|---|
Aspirin | Drug: Aspirin 200 mg tablet | ASPIRIN 200 mg TAB |
Ciprofloxacin | CPX (Ciprofloxacin 250 mg tablet) | CIPROFLOXACIN 500 mg TAB (CPX) |
Amoxicillin | Amoxicillin (125 mg/5 ml) |
|
Name dose formhttp://wiki.ampath.or.ke/pages/viewpage.action?pageId=36569685
Naming of Combination drugs
Split combination drug components by slash '/' , and put them in alphabetical order which is standard methodology used by RxNORM. AMPATH uses the full name of the drug since there are multiple shortname (ie. AZT and ZDV).
For example:
- Lamivudine 150 MG / Nevirapine 200 MG / Zidovudine 300 MG Oral Tablet
- 3TC 150mg / AZT 300 mg / NVP 200mg
Include the route in the drug name (ie. oral, IV, IM, topical,inhalation drug, etc). In the example of ISONIAZID 100 mg TAB, the drug route would be oral, so it should be Isoniqzid 100 mg tablet (oral).
Representation of Drugs
Roger believes that there is a fair level agreement that there are four levels of granularity in the classification of drugs for EMR purposes. Level 1 (most general) is a category (e.g. Pain Medication, HIV Anti-retrovirals) which is used to narrow the universe of drugs. Level 2 is the active ingredient(s) which the drug contains, which is what the doctor is trying to specify for the patient. Level 3 is level 2 plus strength (the amount or concentration of each active ingredient in the drug) and form (tablet, liquid, intravenous fluid, etc.). This is what should be on the prescription, along with quantity, frequency, refills and other instructions; this should be stored in the EMR. Level 4 is level 3 plus manufacturer and lot number. This is what the pharmacist/nurse actually dispenses/administers and should be recorded in the pharmacy system (outpatient) or drug administration record (inpatient). Level 3 is the level at which a facility's formulary (the list of drugs it keeps available) is specified. Levels 2 and 3 are based on the organization of a standard nomenclature known as RxNorm, and other standard vocabularies also focus on these levels.
Names in the standard vocabularies are specified as chemical names (e.g. at level 2, stavudine/lamivudine/nevirapine), although they may be known at the facility otherwise (e.g. d4T/3TC/NVP or Triomune or Regimen 1a). We try to encourage people to use the standard nomenclature, with other names maintained as synonyms. We do this by guidance in the wiki and by encouraging the use of the MVP concept dictionary. But we don't require it or validate against it. If a facility wants the fully-specified name (at level 3) to be Triomune 30 rather than stavudine/lamivudine/nevirapine 30mg/150mg/200mg, we allow it. If a facility wants to have separate entries for Triomune and stavudine/lamivudine/nevirapine, we allow it. If a facility wants to let the doctor prescribe Regimen 1a (level 2) rather than Regimen 1a(30) or 1a(40) (level 3), we allow it. In general, our approach has been for the most part to allow the clinic to go on doing what it has been doing rather imposing change upon them, even though we (and substantially all the authoritative literature) are in agreement that prescribing at level 3 using standard ingredient names and strengths is the best at reducing drug administration errors.